L-DLPFC - SGC Marker of Depression
Left-Dorsolateral Prefrontal Cortex (DLPFC) - Subgenual anterior Cingulate Cortex (SGC) circuit as a treatment resistant depression response marker in transcranial magnetic stimulation treatment
Neurophysiological Biomarkers for Neuromodulation in Treatment-Resistant Depression
As a neuroscientist driven by the challenge of unraveling depression’s neural circuits, my work has centered on developing circuit-specific biomarkers using transcranial magnetic stimulation combined with electroencephalography (TMS-EEG) to guide and optimize neuromodulation therapies for treatment-resistant depression (TRD). This aligns seamlessly with my research interests in mechanistic neurophysiological markers and targeted interventions. From my postdoctoral at the Centre for Addiction and Mental Health (CAMH) to my role at UC San Diego, where I helped establish the Interventional Psychiatry Program, I have led and contributed to studies validating subgenual cingulate cortex (SGC) hyperactivity and dorsolateral prefrontal cortex (DLPFC)-SGC effective connectivity as pivotal features of TRD. These biomarkers not only illuminate depression’s pathophysiology but also inform personalized treatments with repetitive TMS (rTMS) and potentially other circuit spesific interventions Looking ahead, I envision extending this to accelerated intermittent theta-burst stimulation (aiTBS) protocols, integrating multimodal data for targeting, to advance clinical outcomes. A cornerstone of my research is the theoretical anticorrelation between the left DLPFC and SGC, where rTMS treatment directed to the DLPFC may modulate SGC activity transsynaptically, normalizing hyperactivity as measured by TMS-EEG and linked to depressive symptoms.
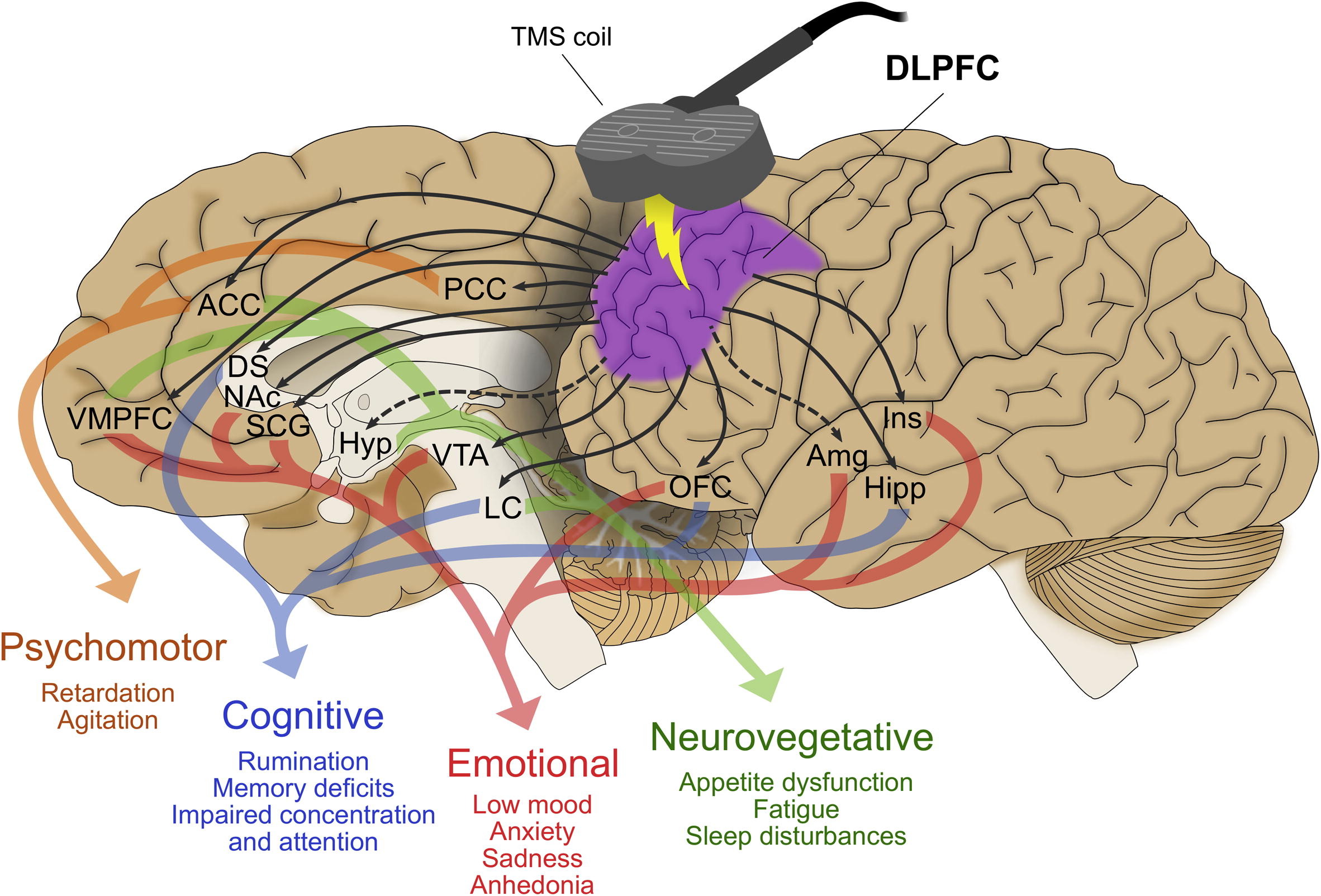
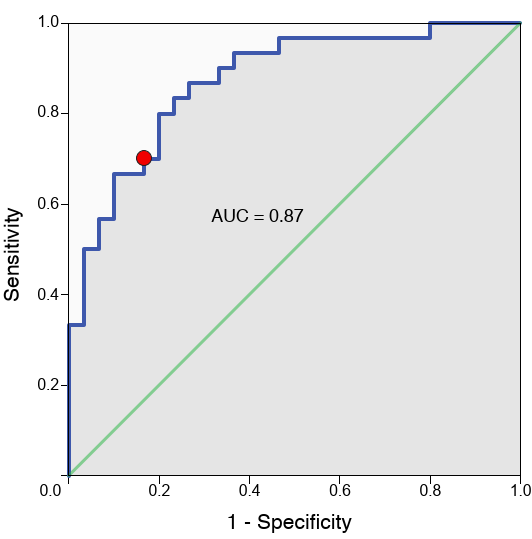
In an early study I led, we used TMS-EEG to probe SGC hyperactivity and DLPFC-SGC effective connectivity in TRD patients before and after high-frequency rTMS (Hadas et al., 2019). Quantifying SGC activity via significant current density (SCD) and connectivity via significant current scattering (SCS), we found elevated baseline measures in TRD versus controls, with SCD at the subgenual cortex classifying TRD at 77% accuracy. Active rTMS reduced these toward normal levels, correlating baseline SCD with Hamilton Rating Scale for Depression (HRSD-17) scores (ρ = 0.41, P = .03) and SCS changes with symptom relief (ρ = 0.58, P = .047). This was among the first demonstrations of TMS-EEG capturing causal connectivity shifts, positioning DLPFC-SGC dysregulation as a viable therapeutic target.
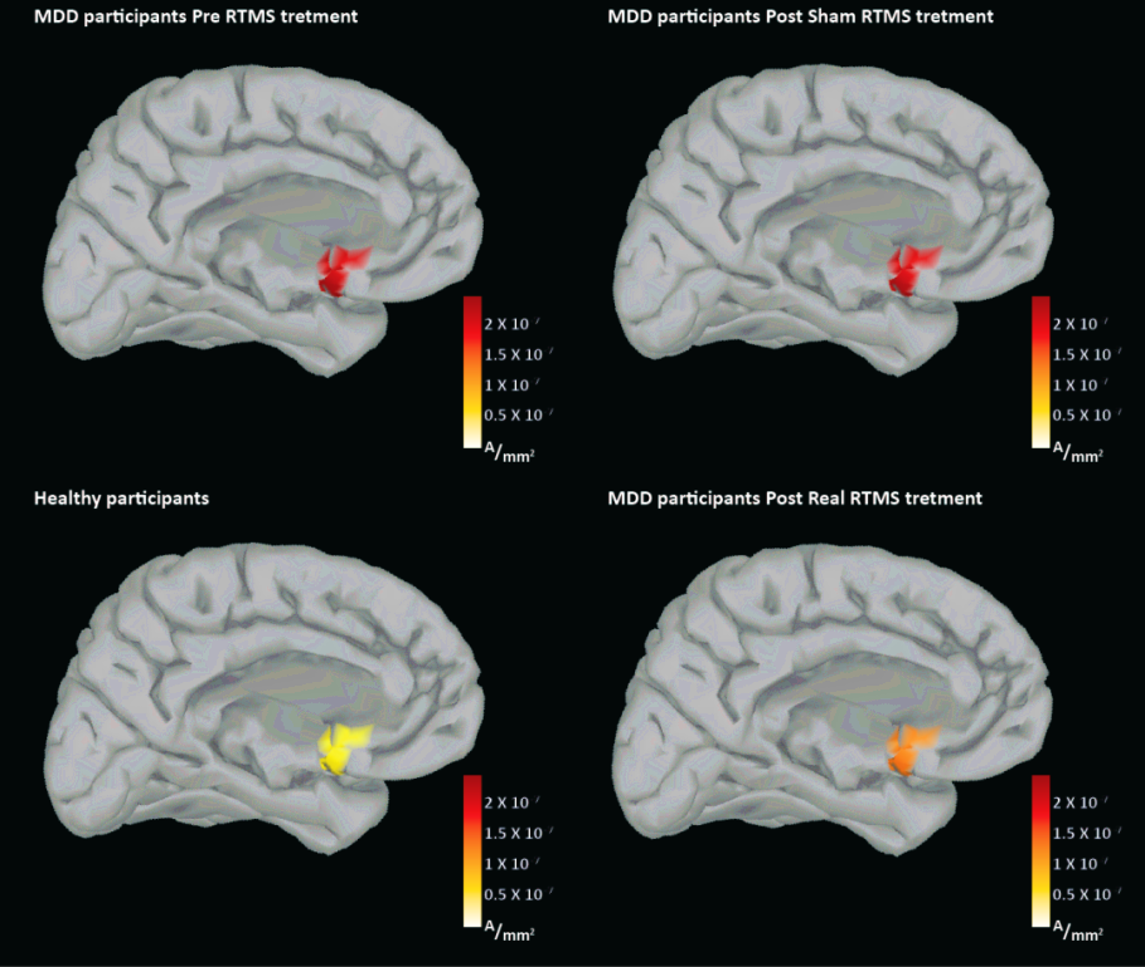
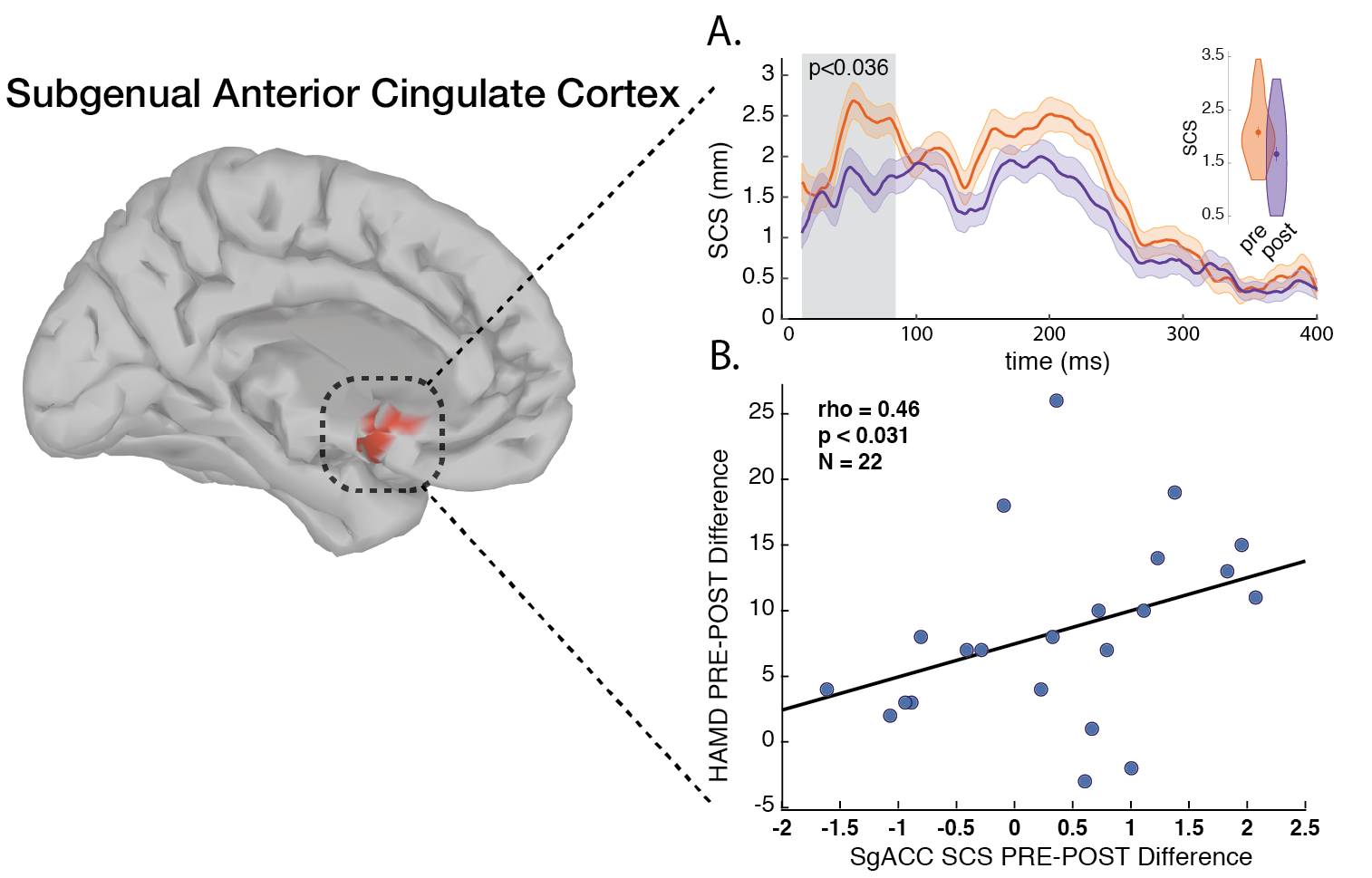
Extending this biomarker to Magnetic Seizure Therapy (MST), a seizure inducing neuromodultory intervention for TRD with reduced cognitive side effects when compared to Electroconvulsive Therapy (ECT), I spearheaded a study showing post-MST reductions in DLPFC-SGC effective connectivity (P < .036), correlating with HRSD-24 improvements (ρ = 0.46, P < .031). Hippocampal (Hipp) localized activation also decreased (P < .015), linking to Montreal Cognitive Assessment (MoCA) changes (ρ = -0.59, P < .026). This dissociated efficacy mechanisms (SGC-DLPFC connectivity) from cognitive impacts (Hipp activation), underscoring TMS-EEG’s value for treatment monitoring symptoms and side-effects spesific biomarkers production (Hadas et al., 2020).
Correlation between fMRI functional connectivity and source localized TMS-EEG activation at the DLPFC-SGC circuit
Building on these foundations, I contributed to a multimodal study integrating resting-state functional MRI (RSFC) with TMS-EEG in 42 TRD patients undergoing individualized aiTBS (Sun et al., 2025; Sun et al., 2025). We found that more negative baseline RSFC between the DLPFC target and SGC correlated with smaller (less negative) N100 amplitudes (Spearman’s ρ = -0.37, P = 0.02) and reduced cortical evoked activity (CEA) in the SGC (ρ = 0.29, P = 0.07). This suggests stronger DLPFC-SGC anticorrelation reflects enhanced inhibitory control, allowing DLPFC-targeted TMS to better suppress SGC activity. Reconciling prior findings—where higher SGC TMS-EEG activation signals worse depression, but negative RSFC predicts better outcomes—this work bridges modalities, supporting personalized targeting without invasive methods. The The manuscript is in progress!!
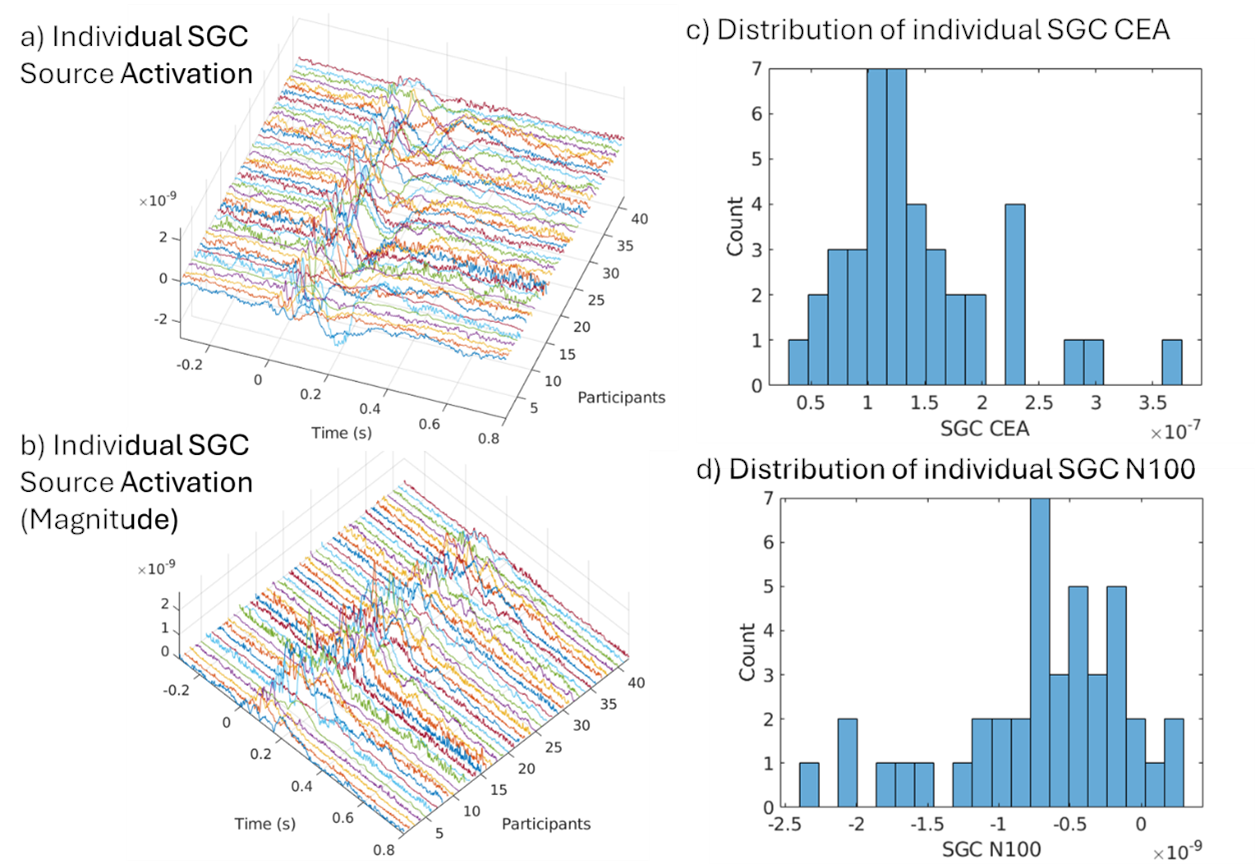
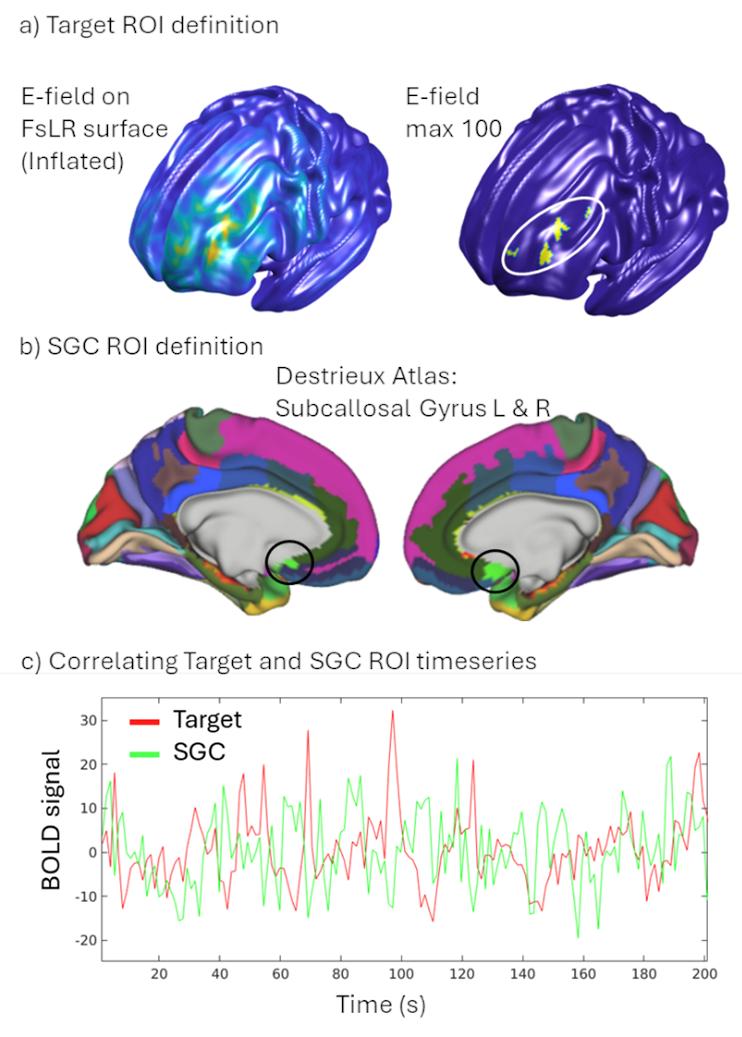
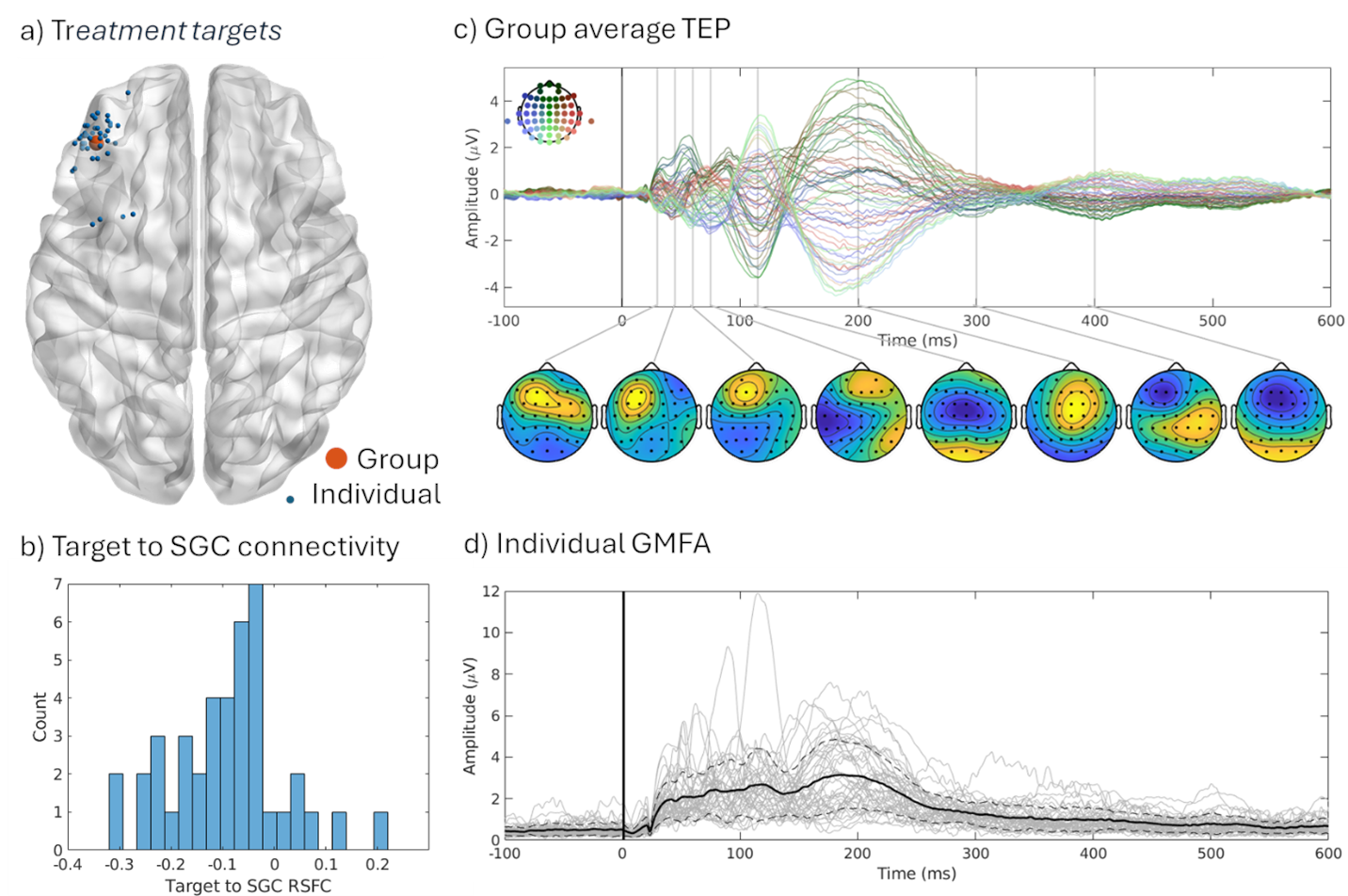
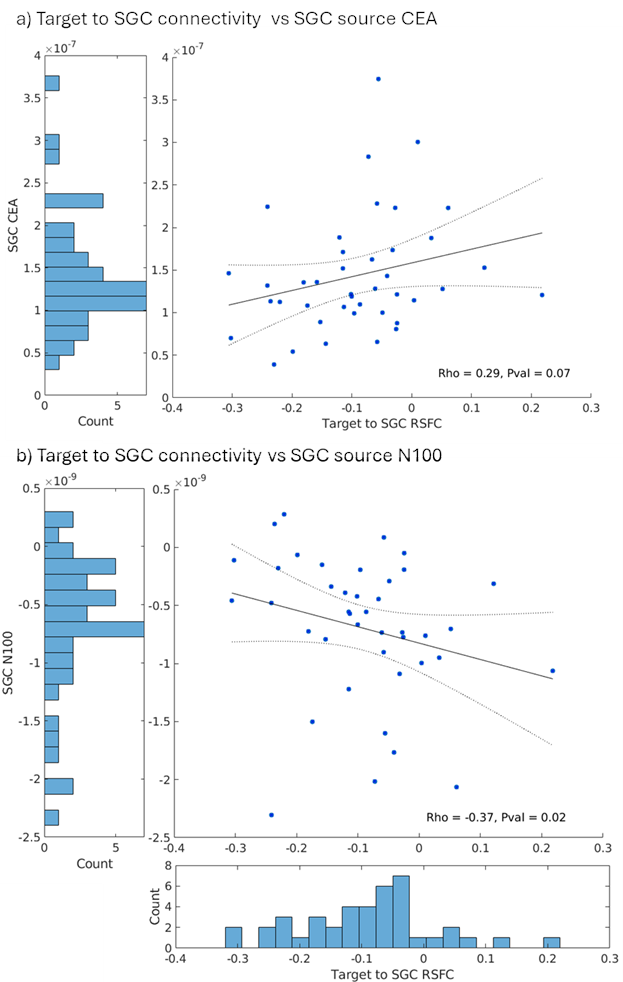
These projects, where I served as first author on the foundational TMS-EEG studies and collaborator on multimodal extensions, highlight my proficiency in biomarker pipelines—from data acquisition to analysis. They fuel my future directions for clinically applicable TMS-EEG biomarkers for depression. By translating these insights from bench to bedside, including my consulting at Deliberate.ai and Salma Health for AI-driven diagnostics, I aim to revolutionize TRD care through innovative, cross-disciplinary science.
References
2025
- In progress: Subgenual cingulate TMS-EEG response from dorsolateral prefrontal transcranial magnetic stimulation relates to the functional connectivity between the two regions in patients with treatment resistant depressionJan 2025