Circuit-Targeted TMS for Bipolar
Accelerated Intermittent Theta-Burst Stimulation (aiTBS) for Bipolar Depression - A Personalized Brain Circuit-Targeted Intervention
Accelerated Intermittent Theta-Burst Stimulation (aiTBS) for Bipolar Depression: A Personalized Brain Circuit-Targeted Intervention
As a neuroscientist deeply committed to advancing targeted treatments for mood disorders, I was excited to lead a clinical trial funded by the Milken Institute Baszucki Brain Research Grant, aimed at developing a novel brain-stimulation intervention for depressive symptoms in bipolar disorder. Bipolar depression remains a challenging condition, often resistant to conventional therapies, and my work has long focused on leveraging non-invasive neuromodulation to engage specific brain circuits implicated in emotional dysregulation. I was awarded to conduct this study by the Baszucki Brain Research Fund and the Milken Institute (Baszucki Awardee list 2022), to test accelerated intermittent theta-burst stimulation (aiTBS)—a high-dose rTMS protocol—delivered over five days targeting the left dorsolateral prefrontal cortex (DLPFC) functionally associated with the subgenual cingulate cortex (SGC) as measured individually for each patient. Our approach integrated cutting-edge computational targeting to optimize stimulation precision, drawing on methodologies from two leading research groups. To ensure individualized targeting, we combined functional magnetic resonance imaging (fMRI)-derived connectivity mapping with finite-elemets model of electric field (E-field). Personalized DLPFC targets were identified using a fMRI connectivity-guided algorithm developed by Cash et al. (2021), which pinpoints clusters with maximal anti-correlation to the subgenual cingulate cortex - a key hub in mood regulation often dysregulated in depression. Cash’s computation enhances reproducibility and precision, achieving left-DLPFC target localization within ~2 mm across sessions Cash et al. (2021). We then optimized coil placement and stimulus intensity using the Targeting and Analysis Pipeline (TAP) algorithm from Duke University (Dannhauer et al., 2022), which accounts for individual anatomy, hair thickness, and E-field distribution to maximize stimulation at the target.
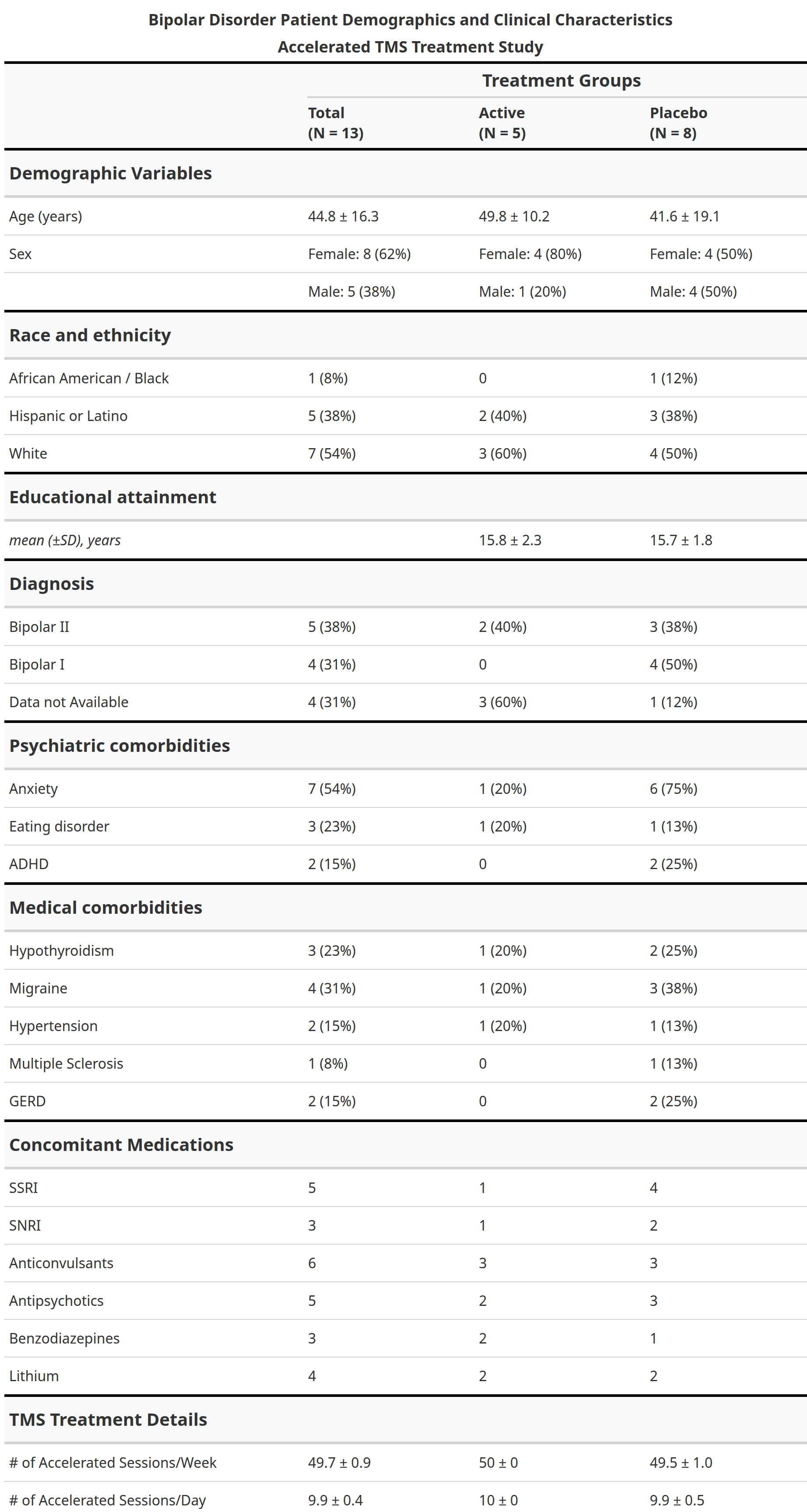
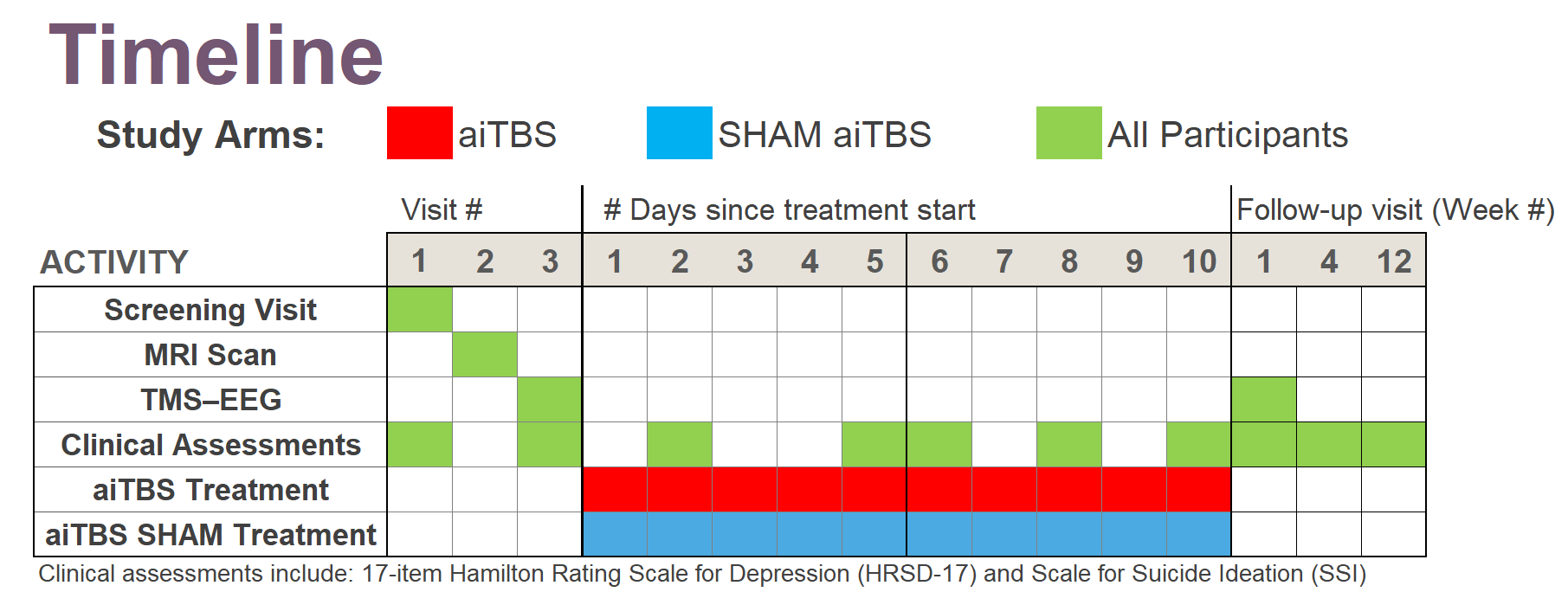
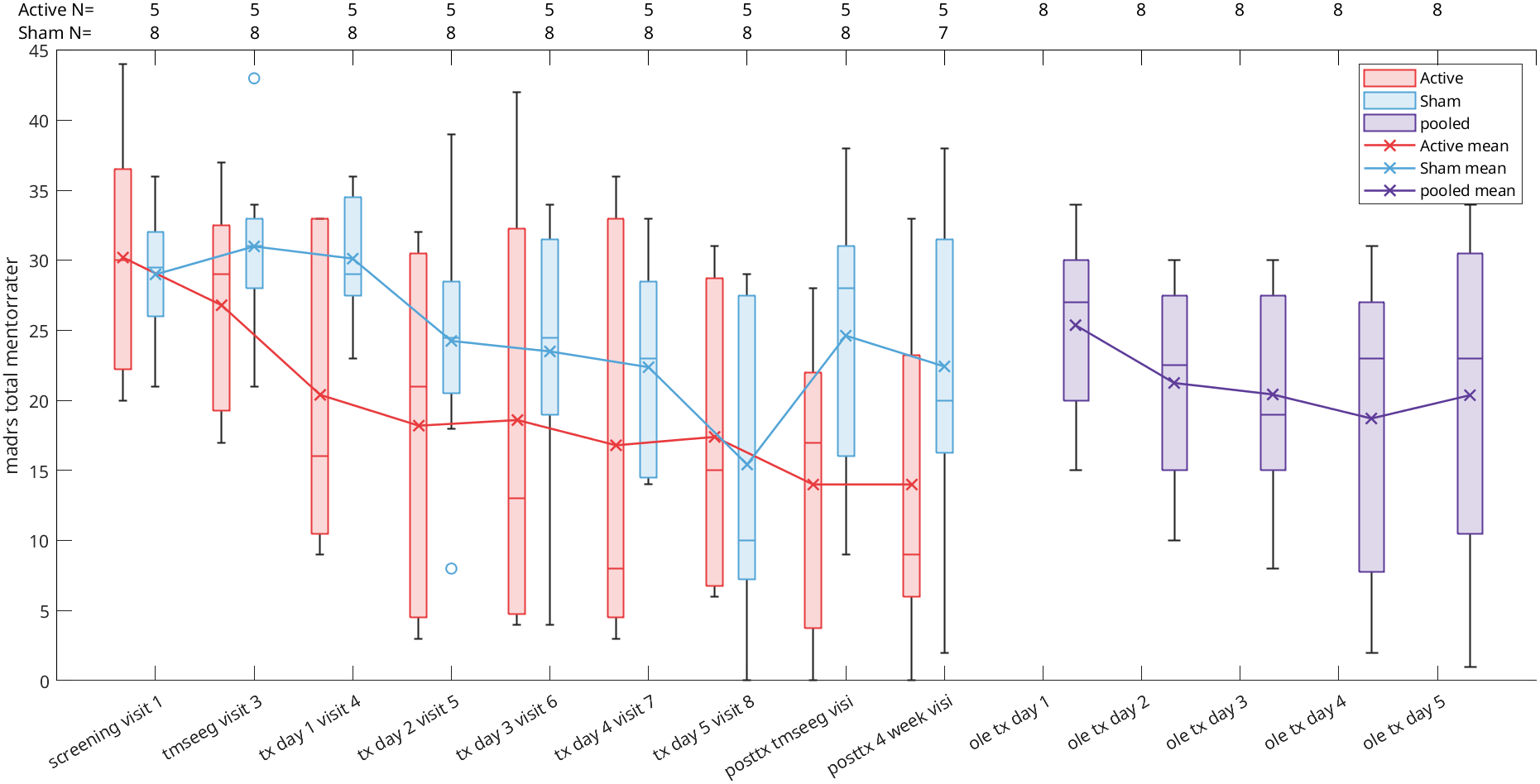
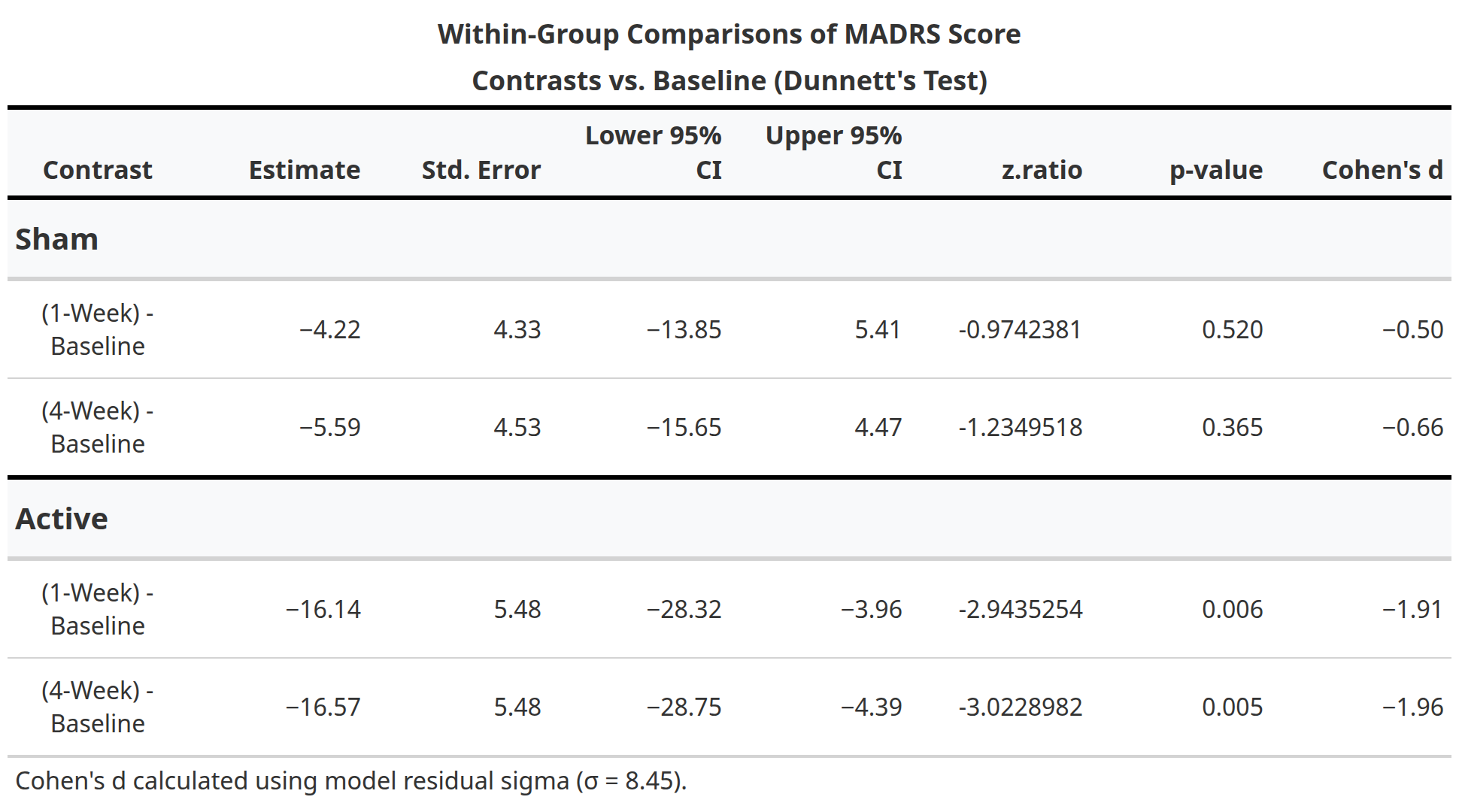
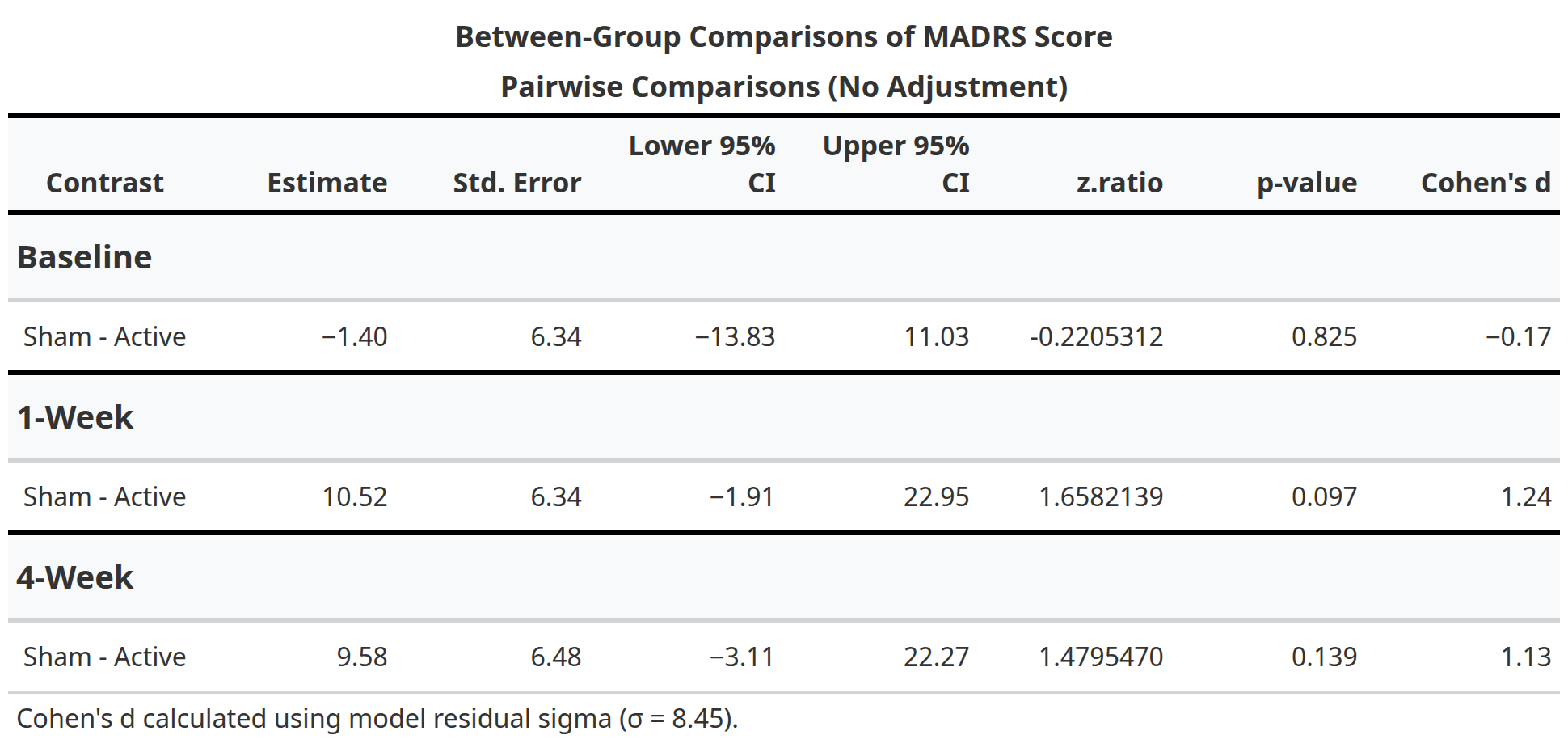
In this randomized, double-blind, sham-controlled trial (NCT05393648), 13 participants with treatment-resistant bipolar depression received either active aiTBS (n=5) or sham stimulation (n=8). Stimulation was delivered in 10 hourly sessions per day for five days, neuronavigated to the personalized targets. The primary outcome was change in Montgomery-Åsberg Depression Rating Scale (MADRS) scores from baseline to one-week follow-up. While between-group differences did not reach statistical significance due to the small sample size, active aiTBS produced substantial within-group improvements: MADRS scores decreased by 56.8% at one week and 55.2% at four weeks (p < 0.01), with 60% of active participants achieving response (≥50% reduction). Sham effects were minimal (12.2% and 20.0% reductions, respectively). No manic switches occurred, supporting aiTBS safety in bipolar populations (Appelbaum et al., 2025). This trial builds on prior evidence for accelerated TMS in unipolar depression (e.g., Cole et al., 2020) and aligns with my broader goal of translating circuit-level biomarkers into clinically viable tools for precision psychiatry. By validating aiTBS in bipolar depression and demonstrating the feasibility of advanced targeting, this work paves the way for larger trials and multimodal biomarkers integrating TMS-EEG, fMRI, and behavioral metrics—areas I plan to expand in future research to predict and enhance treatment response in mood disorders.
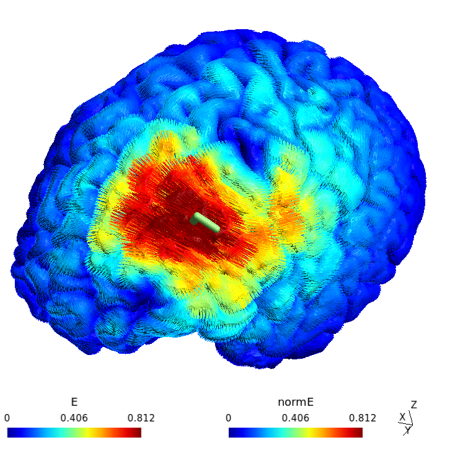
Cortical surface rendering of a participant’s cortical E-field distribution during aiTBS, optimized via the TAP algorithm (Dannhauer et al., 2022). Warmer colors indicate higher E-field strength (V/m), focused at the personalized DLPFC target. This modeling ensured a more precise dosing at 90% resting motor threshold, accounting for individual head anatomy.
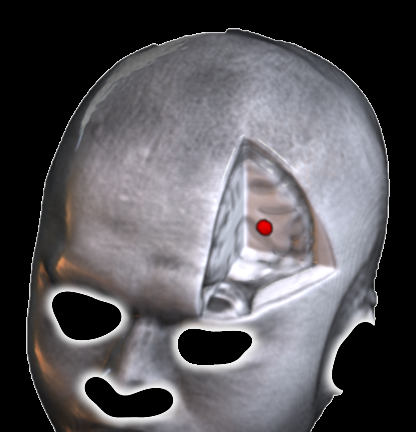
Exemplary aparticipant 3D MRI view showing the computed personalized DLPFC target, selected for maximal anti-correlation with the subgenual cingulate cortex using the Cash et al. (2021) algorithm. This connectivity-based approach ensures stimulation engages depression-relevant circuits.
Github repostory for clinical figures, tables and statistics : UCSD-IPP/Bipolar_milken_analysis
Supported by: Baszucki Brain Research Fund Grant Awardees 2022


References
2025
- Accelerated Intermittent Theta-Burst Stimulation for Treatment-Resistant Bipolar Depression: A Randomized Clinical TrialJAMA Network Open, Feb 2025